AURYXIA has a proven safety profile
Explore the safety and tolerability profile for AURYXIA to see if it is right for your adult patients.1
Safety and tolerability profile evaluated in a pivotal clinical trial1
AURYXIA had a similar tolerability profile as placebo during the 16-week randomized period1,2
| Discontinuation rates due to adverse reactions1 | AURYXIA (N=117) | PLACEBO (N=116) |
|---|---|---|
| 12 (10%) | 10 (9%) |
- The most common adverse reaction leading to discontinuation of AURYXIA was diarrhea at 2.6%1
- The most common adverse event was diarrhea (20.5%), the majority of which was mild to moderate in severity2,3
Safety and tolerability profile evaluated in two clinical trials1*
| Adverse reactions reported in at least 5% of patients treated with AURYXIA | AURYXIA % (N=190) | Placebo % (N=188) |
|---|---|---|
| Any Adverse Reaction | 75 | 62 |
| Metabolism and Nutrition Disorders | ||
| Hyperkalemia | 5 | 3 |
| Gastrointestinal Disorders | ||
| Discolored Feces | 22 | 0 |
| Diarrhea | 21 | 12 |
| Constipation | 18 | 10 |
| Nausea | 10 | 4 |
| Abdominal Pain | 5 | 2 |
*Data from a study of 117 patients treated with AURYXIA and 116 patients treated with placebo in a 16-week, randomized, double-blind period and a study of 75 patients treated with AURYXIA and 73 treated with placebo in a 12-week randomized double-blind period. Dosage regimens in these trials ranged from 210 mg to 2,520 mg of ferric iron per day, equivalent to 1 to 12 tablets of AURYXIA.
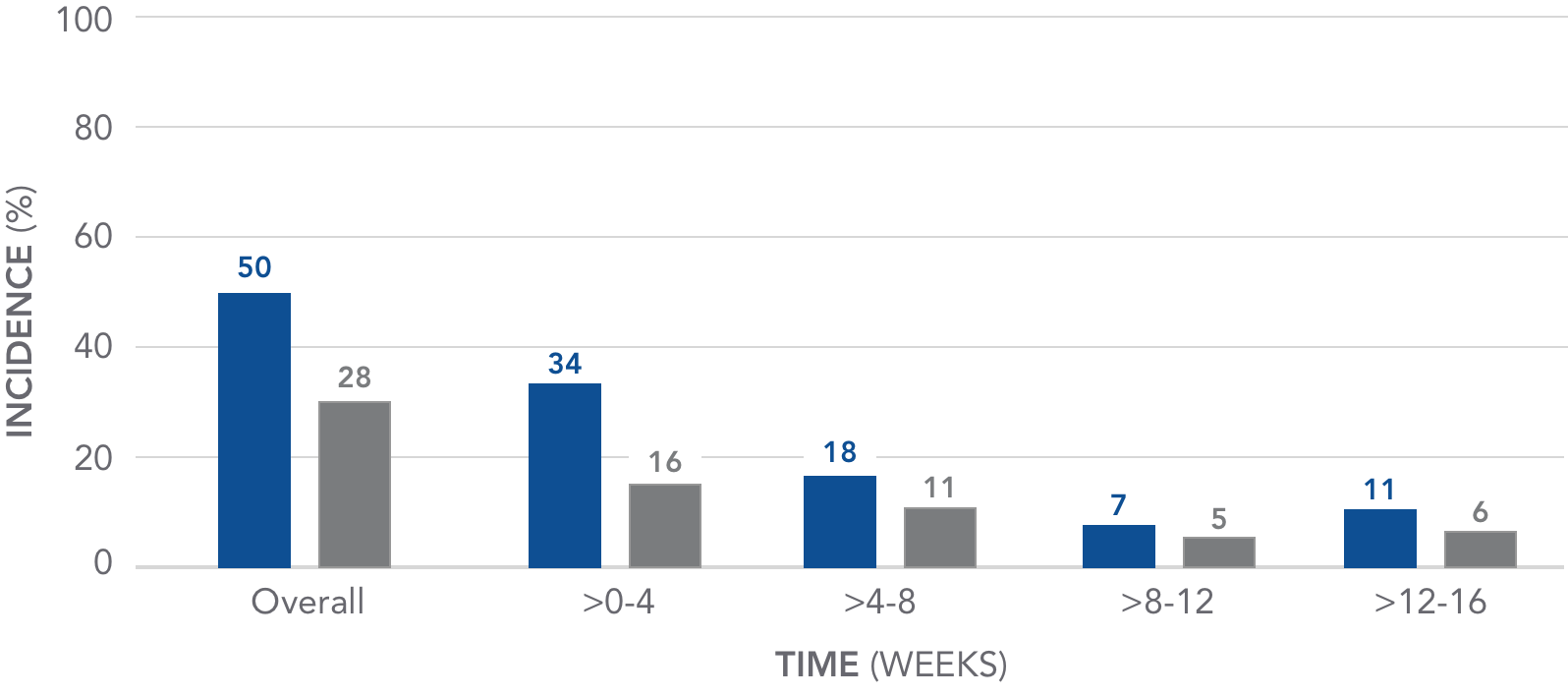
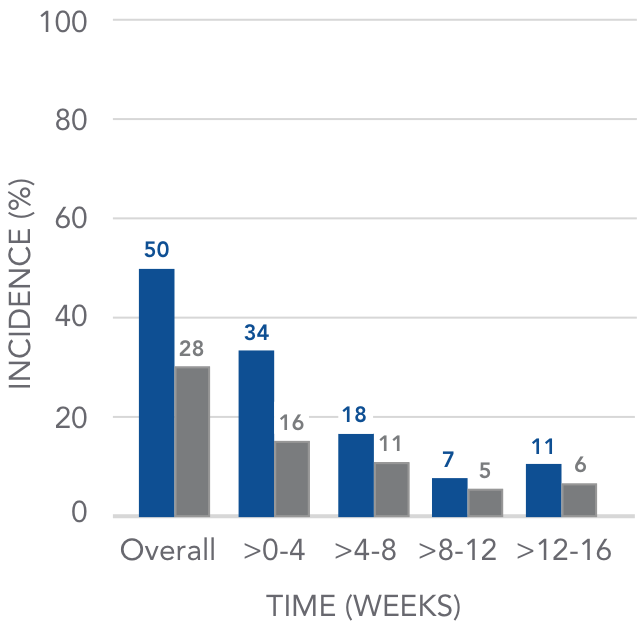
The incidence of GI-related adverse events declined over time
Pooled analysis of GI AEs over 16 weeks4
AURYXIA (N=190)
PLACEBO (N=188)
Safety and tolerability profile evaluated in a pivotal clinical trial1
Similar incidence of severe adverse events (SAEs) between AURYXIA and placebo groups during the 16-week randomized period1,2
No individual SAEs were observed in more than 5% of patients treated with AURYXIA.
| SAEs during the 16-week randomized period2 | AURYXIA (N=117) | PLACEBO (N=116) |
|---|---|---|
| 14 (12.0%) | 13 (11.2%) |
You may also be interested in:
Trial design1
In a 24-week study consisting of a 16-week, randomized, double-blind, placebo-controlled efficacy period followed by an 8-week, open-label safety extension period, this trial evaluated the efficacy and safety of AURYXIA for the treatment of iron deficiency anemia in adult patients with CKD not on dialysis. Patients who were intolerant of or have had an inadequate therapeutic response to oral iron supplements, with hemoglobin ≥9.0 g/dL and ≤11.5 g/dL, serum ferritin ≤200 ng/mL, and TSAT ≤25% were enrolled. Patients were randomized to treatment with either AURYXIA (n=117) or placebo (n=117).
The primary endpoint was the proportion of patients achieving a ≥1.0 g/dL increase in hemoglobin at any time point during the 16-week efficacy period. Use of oral iron, IV iron, or ESAs was not permitted at any time during the trial.
AE=adverse event; CKD=chronic kidney disease; GI=gastrointestinal; SAE=serious adverse event.
