Safety: Pharmacodynamics in AURYXIA
AURYXIA has been shown to increase iron parameters, including TSAT and ferritin1
- AURYXIA has a warning for iron overload which may lead to excessive elevation in iron stores; serum ferritin and TSAT should be assessed prior to initiating and monitored while on therapy1*
- During the 52‐week active‐controlled period in the Phase III trial in which concomitant use of IV iron was permitted, gradual increases in iron parameters occurred over the first 3 to 6 months and then plateaued1,2
Assess iron parameters prior to initiating AURYXIA as a phosphate binder and monitor while on therapy1
AND
In patients receiving IV iron, a reduction in dose or discontinuation of IV iron therapy may be required1
Mean TSAT levels for AURYXIA vs Active Control1,3*
AURYXIA (n=252)
ACTIVE CONTROL (n=137)
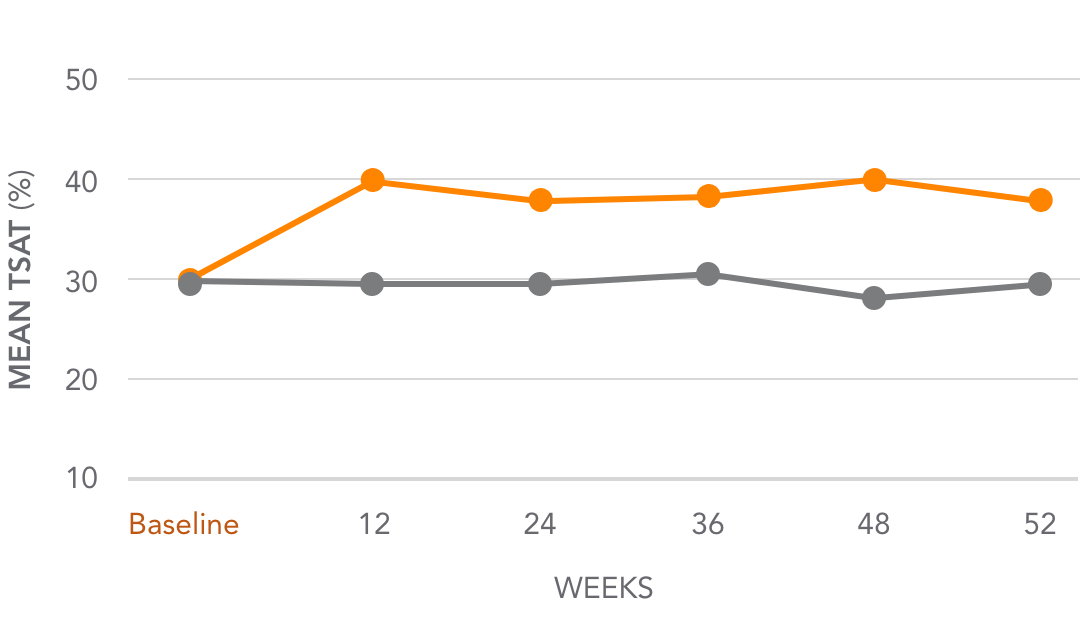
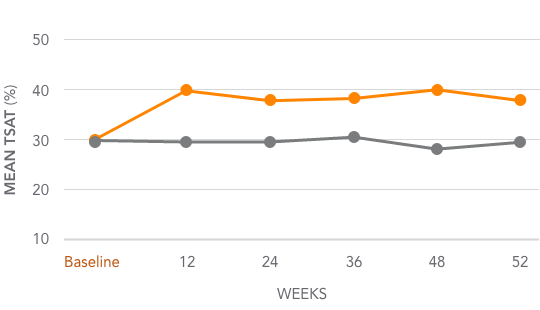
Mean TSAT increased from 31.3% at baseline to 39.2% at Week 52 (~8%)
*Active Control=sevelamer carbonate and/or calcium acetate.
Mean serum ferritin levels for AURYXIA vs Active Control1,3*
AURYXIA (n=253)
ACTIVE CONTROL (n=137)
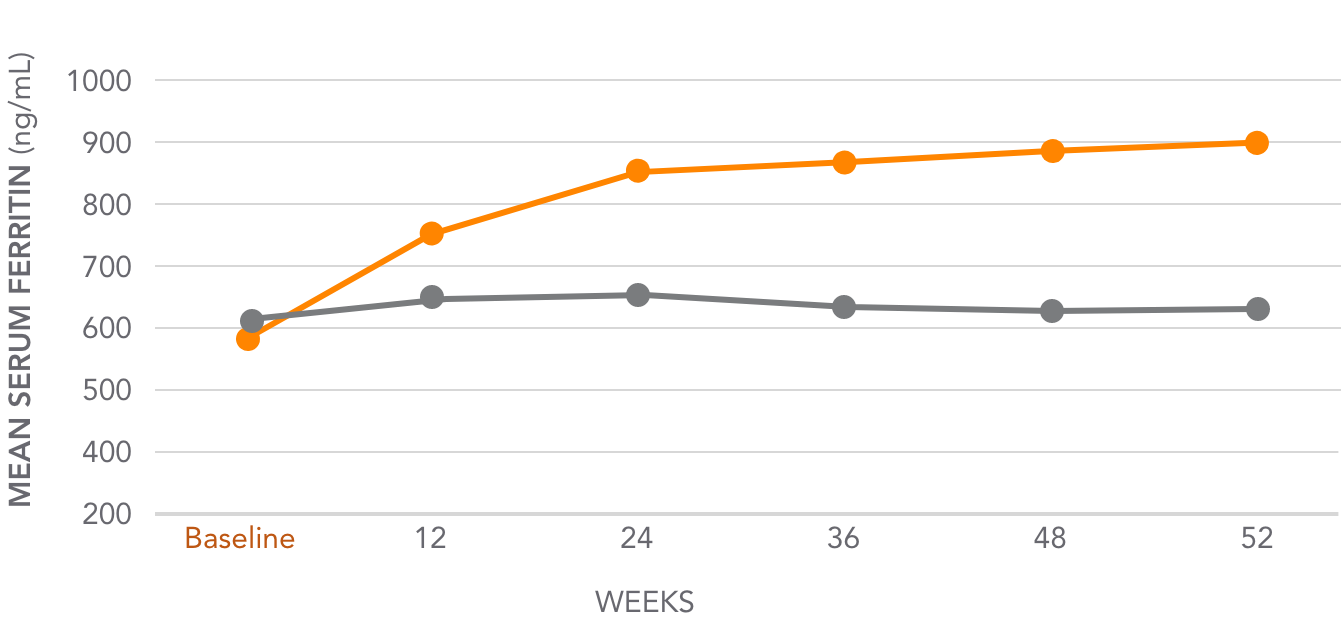
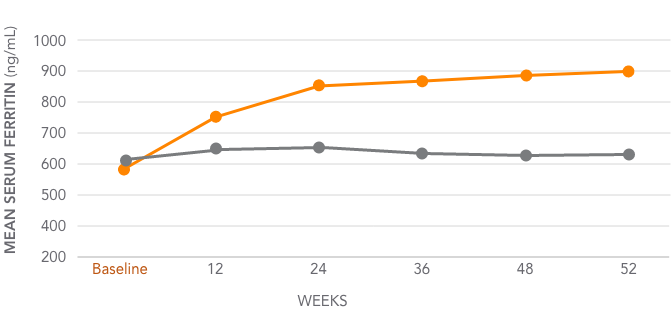
Mean serum ferritin increased from 593 ng/mL at baseline to 895 ng/mL at Week 52 (302 ng/mL)
*Active Control=sevelamer carbonate and/or calcium acetate.
Considerations when evaluating iron parameters
- Is the patient on concomitant IV iron that may affect lab results?
- If the patient is not on IV iron, when was the last dose?
- Have they been off IV iron for an extended period of time?
- Do you look at trends of TSAT and/or ferritin increases when you stop IV iron?
- Was there a recent/current inflammatory event that impacted iron parameters?
- Is the lab value being impacted by the timing of the blood draw?
See how AURYXIA helped patients reach their target goals
AURYXIA helped patients reach and stay in the range of 3.5-5.5 mg/dL during a 56-week trial.1
Patients had a mean serum phosphorus level of 7.41 mg/dL at baseline and 4.88 mg/dL at Week 56.4
See trial design

Your partner in helping patients access the medication they need.
Connect with a personal Case Manager today!
AkebiaCares personal Case Managers are standing by live to answer your call and help your patients find coverage.
- 1-833-4AKEBIA (425-3242)
- Monday - Friday
- 8AM ‐ 8PM EST
You may also be interested in:
Trial design1,6
A multicenter, randomized, open‐label trial evaluated the ability of AURYXIA to lower serum phosphorus in patients with CKD on dialysis over 56 weeks. Eligible patients had serum ferritin <1000 ng/mL, serum TSAT <50%, and serum phosphorus ≥2.5 and ≤8.0 mg/dL at the screening visit. The safety and efficacy of AURYXIA were studied in the 52‐week active‐controlled period (AURYXIA n=292, Active Control n=149), then AURYXIA patients were re‐randomized to either continue AURYXIA treatment or receive placebo during the placebo‐controlled period, weeks 52‐56 (AURYXIA n=96, placebo n=96). The primary endpoint was the change in serum phosphorus from baseline (Week 52) to Week 56 between AURYXIA and placebo. The key secondary endpoint was the change in serum phosphorus from baseline (Week 0) to Week 52 between AURYXIA and Active Control.
CKD=chronic kidney disease; TSAT=transferrin saturation; Active Control=sevelamer carbonate and/or calcium acetate.