Strong and sustained phosphorus reductions for more than a year of treatment (56 weeks)1,2
AURYXIA was effective in getting patients to goal when used as a monotherapy1,2
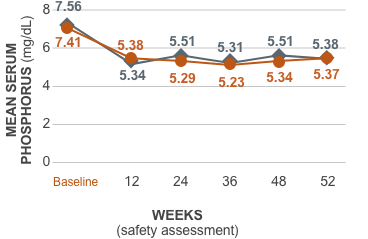
AURYXIA had similar reductions in serum phosphorus compared to Active Control1,2*
Secondary endpoint: treatment difference of 0.02 mg/dL at Week 52 (P=0.89)
AURYXIA (n=292)
Active Control (n=149)
AURYXIA Arm Re-randomization
AURYXIA maintained significant reductions in serum phosphorus compared to placebo1,2
Primary endpoint: treatment difference of -2.18 mg/dL at Week 56 (P<0.0001)
AURYXIA (n=96)
Placebo (n=96)
AURYXIA Arm Re-randomization
*Active Control=sevelamer carbonate and/or calcium acetate.
AURYXIA maintained mean serum phosphorus levels between 3.5-5.5 mg/dL after a year of treatment (up to 56 weeks)2
Patients on AURYXIA had a mean serum phosphorus level of 7.41 mg/dL at baseline and 4.88 mg/dL at Week 562
VIEW DATA TABLES
Ferric citrate had similar reductions in serum phosphorus compared to Active Control2*
| FERRIC CITRATE | ACTIVE CONTROL | ||
|---|---|---|---|
| Mean Serum Phosphorus (mg/dL) | Mean Serum Phosphorus (mg/dL) | Change From Baseline: Treatment Difference P-Value | |
| Baseline (n) | 7.41 (281) | 7.56 (146) | |
| Week 12 (n) | 5.38 (281) | 5.34 (146) | 0.65 |
| Week 24 (n) | 5.29 (281) | 5.51 (146) | 0.24 |
| Week 36 (n) | 5.23 (281) | 5.31 (146) | 0.76 |
| Week 48 (n) | 5.34 (281) | 5.51 (146) | 0.39 |
| Week 52 (n) | 5.37 (281) | 5.38 (146) | 0.89 |
*Active Control=sevelamer carbonate and/or calcium acetate.
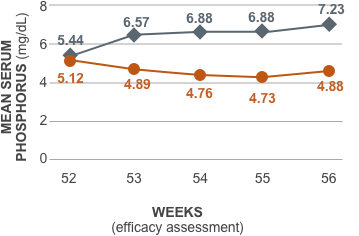
Ferric citrate maintained significant reductions in serum phosphorus compared to placebo1,2
| FERRIC CITRATE | PLACEBO | ||
|---|---|---|---|
| Mean Serum Phosphorus (mg/dL) | Mean Serum Phosphorus (mg/dL) | Change From Baseline: Treatment Difference P-Value | |
| Week 52 (n) | 5.12 (91) | 5.44 (91) | |
| Week 53 (n) | 4.89 (81) | 6.57 (87) | <0.0001 |
| Week 54 (n) | 4.76 (90) | 6.88 (90) | <0.0001 |
| Week 55 (n) | 4.73 (91) | 6.88 (91) | <0.0001 |
| Week 56 (n) | 4.88 (91) | 7.23 (91) | <0.0001 |
SEE TRIAL DESIGN
Trial design1,3
A multicenter, randomized, open‐label trial evaluated the ability of AURYXIA to lower serum phosphorus in patients with CKD on dialysis over 56 weeks. Eligible patients had serum ferritin <1000 ng/mL, serum TSAT <50%, and serum phosphorus ≥2.5 and ≤8.0 mg/dL at the screening visit. The safety and efficacy of AURYXIA were studied in the 52‐week active‐controlled period (AURYXIA n=292, Active Control n=149), then AURYXIA patients were re‐randomized to either continue AURYXIA treatment or receive placebo during the placebo‐controlled period, weeks 52‐56 (AURYXIA n=96, placebo n=96). The primary endpoint was the change in serum phosphorus from baseline (Week 52) to Week 56 between AURYXIA and placebo. The key secondary endpoint was the change in serum phosphorus from baseline (Week 0) to Week 52 between AURYXIA and Active Control.
CKD=chronic kidney disease; TSAT=transferrin saturation; Active Control=sevelamer carbonate and/or calcium acetate.
SEE ADDITIONAL DATA POINTS
Percentage of patients achieving serum phosphorus levels of ≤5.5 mg/dL4†
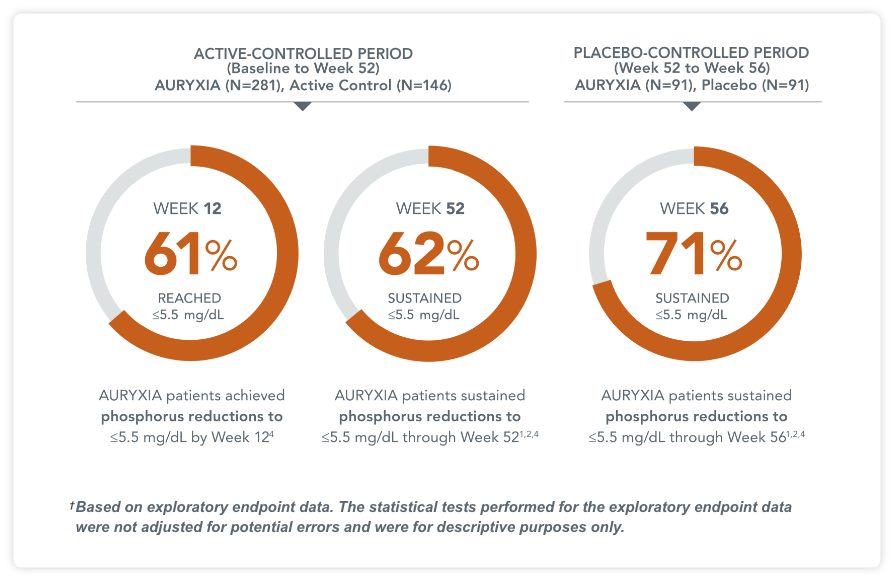
ACTIVE CONTROLLED PERIOD (Baseline to Week 52) AURYXIA (n=281), Active Control (n=146)
AURYXIA patients achieved phosphorus reductions to ≤5.5 mg/dL by Week 124
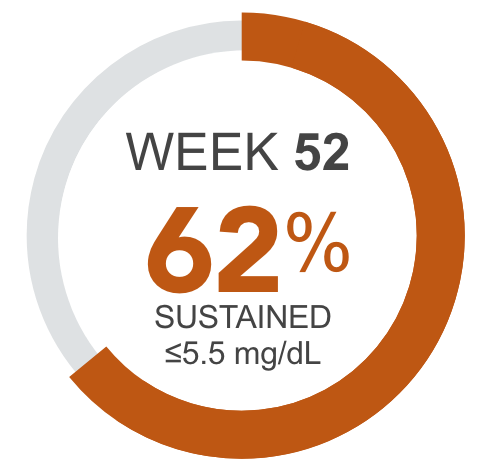
AURYXIA patients sustained phosphorus reductions to ≤5.5 mg/dL through Week 521,2,4
PLACEBO CONTROLLED PERIOD (Week 52 to Week 56) AURYXIA (n=91), Placebo (n=91)
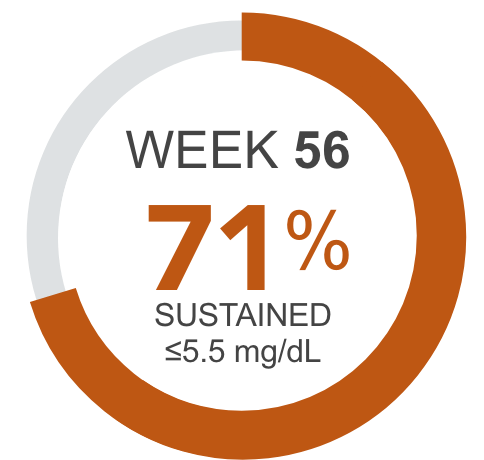
AURYXIA patients sustained phosphorus reductions to ≤5.5 mg/dL through Week 561,2,4
†Based on exploratory endpoint data. The statistical tests performed for the exploratory endpoint data were not adjusted for potential errors and were for descriptive purposes only.
